

Water flowing through a filtration system to clean, then balanced and sanitized is the way.
Balanced so that the sanitizing agent work effectively.
Minerals required to balance the water are measured in parts per million. (ppm)
| Balanced Water |
Raise ppm per 1000l water |
Lower ppm per 1000l water |
|
| Total Alkalinity |
110 80-120 ppm |
Add 15g sodium bicarbonate (baking soda) to raise by 10 ppm |
Add 16ml concentrated hydrochloric acid (pool acid) or 2g dry acid
(sodium bisulphate) to lower by 10 ppm |
| pH | 7.2 7-7.4 |
Add 4g sodium carbonate (soda ash) Ph around 11 or 100g sodium
bicarbonate (baking soda) Ph around 7.8 to raise by 0.1 |
Add hydrochloric acid or dry acid (sodium bisulphate)
according to acid demand test |
| Calcium Hardness | 500 300-600 ppm |
Add 15g Calcium Chloride to raise by 10 ppm |
New water |
| Chlorine Sanitzer | 1.5-3 |
Add 1.5g calcium hypochlorite or 2.8g granualar TCA or 10,3 g liquid chlorine to raise by 1 ppm |
Will lower naturally by sanitizing and sun's uv rays |
| Stabilizer |
15 5-20 ppm |
Add 7.5g cyanuric acid to raise by 10 ppm |
New water |
The Romans not only great engineers in road building with understanding of cement structure and application but great water way and pool builders with their aqueducts and public baths.
They understood how the chemical balance of water affected surrounds. Acidic water leaching from pool enclosures, damaging them, causing leaks. Alkaline water depositing calcium and blocking pipes.
Then in 1804, Scotland a filtering system was developed. Water flowing through holding dams where silt drifted to the bottom for collection then on through stone aggregate then sand filtration. From this the first sand filter was developed and mainly still used.
As for cleaning and sanitizing, ancient Egypt understood the collation effect of alum powder binding matter in water and further sanitized water with the aid of copper and silver. These principles still used today. Onward, chlorine is discovered as a sanitizing agent in 1846 and still used extensively for sanitizing water.
Clear, clean, sanitized pool water require running water through a filtration system to clean, then balanced and sanitized to kill germs and bacteria.
Two Hydrogen atoms sharing electrons with one Oxygen atom via covalent bonds at an angle of 104.45° forms water molecules.
Transparent liquid as a drop. Turquoise, bluish, greenish in color against a white background in volume.
A neutral 7 pH at a temperature of 25o C.
Water molecules strives to a state of equilibrium, balance.
Very soluble, absorbing particles of almost everything. Air, pollen, spores, grass, soil, minerals, metals, human waste, etc. When saturated expelling it to adjoining water molecules and surrounds.
Pure water exposed to air absorbs carbon dioxide from the air which slowly converts into bicarbonate and hydrogen ions that makes the water a little acidic.
Two hydrogen atoms One Oxygen Atom.
Most hydrogen atoms have a nucleus consisting of a proton.
Hydrogen ions spontaneously generate in pure water by dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH-) ions. While the hydroxide ions are kept in solution by their hydrogen bonding with other water molecules, the hydrogen ions, consisting of naked protons, immediately attract to un-ionized water molecules, forming hydronium ions (H3O+). The forming is immediate and on going..........
Generally reference to hydrogen ions and their concentration is as if they were free in this state in liquid water.
An oxygen atom have six electrons in its outer shell, which can hold a total of eight electrons. When an oxygen atom forms a single chemical bond, it shares one of its own electrons with the nucleus of another atom and receives in return a share of an electron from that atom. When bonded to two hydrogen atoms, the outer electron shell of the oxygen atom is filled. Meaning a covelant bond is formed. Two isotopic forms, deuterium and tritium, in which the atomic nuclei also contain one and two neutrons, respectively, are found to a small degree in water.
The polarity of the water molecule plays a major part in the dissolution of ionic, very small particles of matter, hence the amount of dissolved solids found in water.
Water is soluble, dissolve and incorporate other matter without being visible to the human eye.
Particles are absorbed in transparent solution and when saturated expelled to become visible.
Controlling factors, pH and temperature.
pH, the potential of hydrogen or power of hydrogen is a scale measurement that indicates the change in a water, aquas, solution.
a Measure of how acidic or alkaline (basic) the water is at a temperature of 25°C.
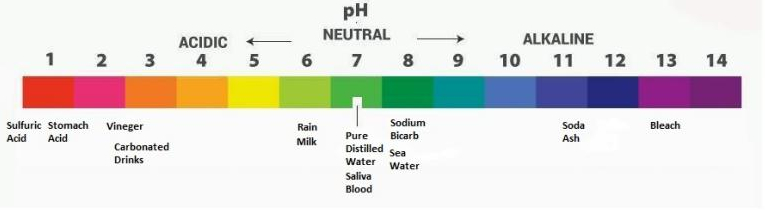
pH range from very acidic 0 to neutral 7 to very alkaline (basic) 14.
Logarithmic units represents the numbers and indicate a 10 times change in the acidity/alkalinity of water. Water at a pH of five is ten times more acidic than water at a pH of six. 100 times more at a pH of of 4.
pH chemically is the measure of the number of free hydrogen and hydroxyl ions in the water.
If the solution contains more hydrogen, hydronium ions (H3O+) than hydroxyl ions (OH-), then the solution is an acid. If the solution contains more hydroxyl ions (OH-) than hydrogen ions (H+), then the solution is a base (Alkaline).
Water with more free hydrogen ions is acidic, attracting, leeching calcium from its surrounds. Water with more free hydroxyl ions is basic, depositing calcium and other matter as metals as surplus on to the surrounds.
Water strives to a neutral state, but is affected by almost everything it comes in contact with. Absorbing matter in the molecule and expelling the matter when saturated. In pools absorbing calcium, metals etc and when too much expelling it.
pH is affected by chemicals and matter in the water.
The pH of water and temperature determines the solubility (amount that can be dissolved in the water) of chemical constituents such as nutrients (phosphorus, nitrogen, and carbon) and heavy metals (lead, copper, cadmium, etc.). Metals are more soluble at a lower pH.
The higher the pH the more matter bound. Then when saturated, expelled out of the water molecule.
Chemicals and every thing else added to water change the pH and thus the efficacy of what you want to achieve in this case safe clean hygienic pool water.
This include the disinfectant agent as chlorine added to the water and how effective it will be.
Temperature Affects pH
Pure water under standard conditions at a temperature of 25°C is neutral
at 7 pH. At 30°C neutral pH is 6.92.
The higher the temperature the more water dissociates into ions, leading
to a higher concentration of hydrogen ions (H3O+) which results in a
lower pH,
This does not mean the water has changed acidity. As per the
Bronsted-Lowry acid-base theory, acids and bases are defined by their
interactions together.
The ratio of products to reactants is expressed as an equilibrium (or
dissociation) constant (K). This constant is affected by
temperature, pressure, and ion concentration of water, with temperature
having the largest effect.
Water can give and receive protons, allowing it to act as both an acid
or base for other compounds, or even for itself in a process called
autoprotolysis.
As stated the pH scale indicate the strength of acids and bases,
7 pH in standard conditions is neutral, meaning the water has an equal
ratio of H3O+OH. Below 7 pH is acidic, meaning there is more H3O+
than OH–, and vice versa above 7 pH. Outside of standard
conditions, these assumptions no longer apply. Pure water at 30°C
has more H3O+ than at 25°C so pH is lower, but an equal amount of OH– is
also present, so the neutral pH point shifts to 6.92. As such,
acidity does not change.
The graph below shows the neutral measurement of pure water (Kw) at
different temperatures and the corresponding shift of neutral pH.
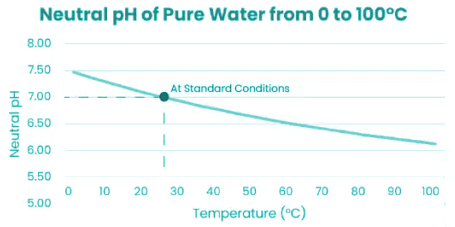
In balancing pool water especially heated pool or spa water be aware of neutral ph being less than 7. So depending on the water temperature your ph goal of 7.2 could be 6.8.
To be clear a certain stable pH in pool water is necessary for pool chemicals to work effectively. In my view a pH of 7.2 at a temperature of 25°. Adjusting this slightly according to the water temperature.
After one session meaning your pump will filter the volume of water, as example 10,000l, 10,000l will be filtered but only approximately 48 % of the original body of water will be filtered. So some of the total body of water will be filtered more and some not at all.
This is important, if the whole body of water is not filtered there are still plenty impurities in the water.A standard pool set up is, the pool, weir (water outlet), piping, water pump, water filter and aimflow (water inlet).
When on, the centrifugal pump pulls the water in the pool through a weir, weir basket and pump basket that traps larger objects as leaves and grass. The water then is pulled to the pump rotor which push it further through the Multi port Valve to a filter that traps smaller particles.
The filtered water is pushed further through the piping and finally through the aimflow (water outlet) back into the pool.
The aimflow gives direction to the flow of the water in the pool. This enables us to direct the fallen matter on the water surface to the weir and therefore filters.
The weir is located at the the top of the pool under the water surface, usually opposite the aimflow. Matter that falls into the water drifts on the surface and will be pushed by the stream of water from the aimflow and pulled through the weir where trapped in the baskets and filter for removal.
If you have a standard cleaner attached to the weir inlet operating in your pool the floating matter will not be sucked through the filters timiously but will drift and sink to the floor where it will be sucked up by the pool cleaner much later. The floating matter, spores, leaves, grass etc left in the water will change the consistency of the water and lead to algae. Especially on warm sunny days with the pump not running and no water flow. Algae which originate from pollen, spores, grass cuttings etc start to grow by way of photosynthesis meaning the sun supply the nutrients.
Good practice would be to use the pool cleaner only once or twice a week depending on your local conditions and leave the weir open to remove floating matter immediately.
The filtering cleans the water and filtered particles trapped in the filter. The filter requires periodical cleaning other wise pressure builds up in the filter and trapped particles pushed into the pool again. That means in case of a sand filter a backwash and rinse weekly.
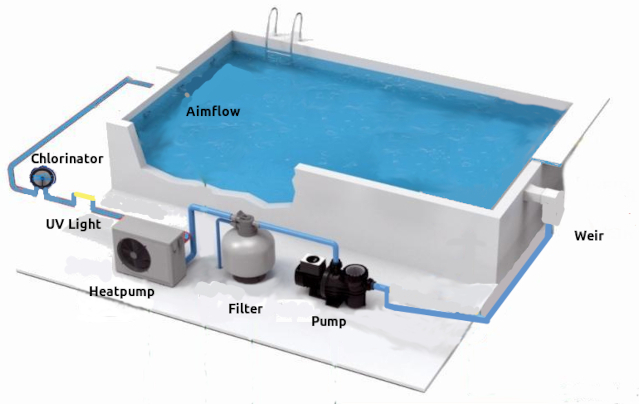
Water enters the filter through the multiport valve from the top. If a sand filter water, flows through the sand where impurities and particles are trapped by the sharp edges of the grains of sand. Water then pass through small finger like slit ted plastic pipes that sand can not pass, back to the pool.
Filtering media is measured by how small the particles of matter trapped.
1micron = 1 millionth of a meter = a thousandth of a millimeter.
Sand filters with silica sand or glass as media, filters and trap 30 to 40 micron particles.
Zeolite in place of sand trap and filters 3 to 6 micron particles with the ability to remove metals from water.
Cleaning the filter media is easy. Backwash and rinse.
Some times sponge like cubes are used in place of other media. Very difficult to clean. Requires removal and washing of the cubes. Difficult and time consuming.
Cartridge filters 5 to 30 microns
Cleaning filters a little more difficult. Remove filters and clean.
Diatomaceous Earth (D E filter) filter 1 to 6 microns
Basically a cartridge filter with a quantity of diatomaceous earth (small sea creature residue) added, that attaches to the cartridge filters to trap even smaller matter.
Cleaning more difficult.
If the filter is not cleaned regularly pressure builds up, water flow is retarded and trapped media spills back into the pool water.
Clean the filter at least once a week.
You need to know the volume of water in your pool.
To determine the size of the pump, filter, chlorinator, heatpump, etc required as this equipment are rated according to the volume of water..
To add the correct amount of chemicals to attain and maintain balanced sanitized water.
An incorrect volume will lead to incorrect dosage, unbalanced water and under performing equipment.
Volume Calculations L = length
W = width
V = volume
r = radius (half of the diameter of a circle) Diameter = Distance between
widest parts of a circle
D = depth (If the pool floor slopes (deep + shallow end ÷ 2)
π = (pi) 3.14 (a factor used in calculations with circles)
Volume of Water
Measure the pool in meters or feet.
Rectangle Volume = L x W x Average Depth (deep + shallow end ÷ 2)
Circular Volume = r2(rxr) x π(3.14) x D
Oval Volume =long diameter x short diameter x average depth x 0.79
You have the volume in cubic meter or feet.
1 cubic meter of water = 1000 liters
1 cubic foot of water = 7.5 gallons
Convert cubic meter to liter = x 1000l
Convert cubic feet to gallon = x 7.5g
Spas: Required turnover every 30 minutes
therefore required flow rate is:
Gallons/ Liter ÷ 30 minutes = min flow rate in g/l per minute
General use, swimming club pools: Required
turnover at least every 6 hours (6 x 60 min = 360 min)
Gallons/Liter ÷ 360 minutes = min flow rate in g/lpm
Limited use pools: Required turnover at
least every 8 hours (8 x 60 min = 480 min)
Gallons/Liter ÷ 480 minutes = min flow rate in g/lpm
The body, volume of water requires filtering 1 to 2 times daily.
The volume of water, the piping setup and type of filter will determine the power and flow rate required of your pump.
Pump specifications usually give a total head maximum measured in feet or meter and by way of graph the flow rate at certain head.
Total head means the pumps ability to push water through pipe to a certain maximum height measured in feet or meter. The more powerful the pump the higher it is able to push the stream of water. Above that height no water flow. A pump working with no flow will cause over heating and a destroyed pump. So usually the more powerful the pump measured in kilowatt Kw the greater the head, the more flow measured per minute or per hour.
Usually pump specifications indicate water displacement measured in liters per minute. From around 260 liters per minute.
Water pressure, flow, is impeded by
friction slowing down flow and therefore a decline in head.
All these flow restrictive components are measured in feet or meter of head.
This balance feet/meter of head enables us to calculate the flow rate of the pump in the setup and if the pump is able to circulate the water through the filter 1 to 2 times a day.The faster the flow the more the head loss
Starting with the piping showing loss of head at different flow rates.
PVC 50 mm pipe 100ft/40m.
| Gpm | HeadLoss | Lpm | HeadLoss |
| 50G | 4ft | 189L | 1.2m |
| 60G | 5.6ft | 226L | 1.67m |
| 70G | 7.4ft | 264L | 2.2m |
| 80G | 9.5ft | 302L | 2.5m |
| 90G | 11.8ft | 340L | 3.5m |
| 100G | 14.4ft | 378L | 4.3m |
Following calculated at a flow rate of 70gpm / 264Lpm.
PVC 50mm Pipe Fittings Head Loss
| Elbow 90° | 8.6ft | 2.6m |
| Elbow 45° | 2.8ft | .80m |
| Tee | 7.8ft | 2.3m |
| Coupling | 2.1ft | .6m |
Valve Head Loss
| 3-way | 2.4ft | .7m |
| 2-way | 0.8ft | .2m |
| Check valve | 1.8ft | .5m |
| Push pull valve | 16.4ft | 4.9m |
| Multiport valve | 3.3ft | 1m |
Equipment Head Loss
| D.E. filter | 6.1ft | 1.85m |
| Sand filter | 21.5ft | 6.6m |
| Cartridge filter | 16.2ft | 4.9m |
| Skimmer | 3.1ft | .93m |
| Main drain | 1.8ft | .51m |
| Return eyeball | 5.5ft | 1.6m |
| Heater | 9.3ft | 2.8m |
In order to obtain a correct sized pump these restrictions requires calculation. Add these restrictions then subtract the total from total head to calculate the water flow per minute. From this you will determine how many hours it will take to filter the volume of water in your pool.
Calculating the pump size in order to run
the total volume of water in your pool during a session keep in mind
this does not mean that all the water in the pool will be pumped.
Studies show that after a first pass of the volume of water, only 48 % of the body of water will be collected and filtered. After a fourth run that percentage is around 88%. If you have a cleaner operating 24/7 in the pool even less.
Be that as it may keeping costs in mind a .75 kw pump running longer could be more cost and cleaning effective than a 1.1 kw pump running for a shorter period.
These days a solar panel feeding
electrical power to a solar pump is an excellent cost effective option.
Even on cloudy days they work.
The majority operate by way of the cleaner connected to the weir by flexible pipe. When the pool pump is on, water pulled through the cleaner enables the cleaner to move and suck up the the collected debris through to the filters for removal.
They are efficient in cleaning but not in cleaning fast as they do not move in a precise pattern.
For that you require a robotic cleaner. They do not use water suction by pump operation but by an independent 12v electrical motor that is incorporated in the cleaner. They have integrated sensors that will plot the area then proceed to clean precisely, going over the area once. a 50000l pool can be cleaned in 2 to 3 hours.
Use a pool cleaner as you use a vacuum cleaner. Once or twice a week as necessary and then remove it from the pool. Matter and leaves falling on the surface will be timeously collected in the weir basket for removal. Filtering will be more efficient and algae forming curtailed.
The total volume of pool water should be filtered twice daily. As temperature is a major factor for sanitation during summer with higher temperatures, 8 to 12 hours and during winter 4 to 6 hours. The higher the temperature the quicker sanitation agents dissipate and become less effective and the more algae are formed.
If a pool is under cover or indoors the suns uv rays which also cause retardation of sanitation agents and promotes algae growth is not a factor and less sanitation is required. Algae does not form as much in colder water so in those areas a winter hiatus is in order.
Your pool volume in liters, the size of your pump and water placement will enable you to calculate the running time of the pump more precisely.
Remove leaves, berries etc from the weir and pool pump baskets.
Run your pool cleaner once or twice per week depending on local conditions.
Brush the pool wall down if your cleaner does not do that and clean (back wash and rinse) the filter properly. Cartridge filter once a month. DE filter once a month with new filter media.
Cleaning the filter media.
Switch off the pump, depress the multiport valve and turn clockwise to the setting, backwash. Start the pump. At that setting the water enters the filter from bottom loosens trapped particles and flushes it out to waste. Back washing should continue until the glass viewer on the side of the multiport valve shows clear water flowing.
By not back washing the filter media becomes blocked, the water is not filtered properly and particles enter the pool again. Pressure also builds up in the filter which affects the pump operation.
After back washing, switch off the pump, depress the multiport valve clockwise to rinse. Start pump and rinse until the glass viewer shows clear water flowing. The filter media have been cleaned. Switch off the pump depress to filter and normal filtration resumes.
Clean but not yet Sanitized.
For sanitizing, the water requires a sanitizing agent and the water molecules self requires balancing to certain standards at a certain ph for the sanitizer to work effectively.
Pool water require effective sanitation in order to eliminate germs, bacteria and algae.
Before any sanitizing agent as chlorine is used, pool water requires balancing as all pool sanitizing chemicals works best in balanced water.
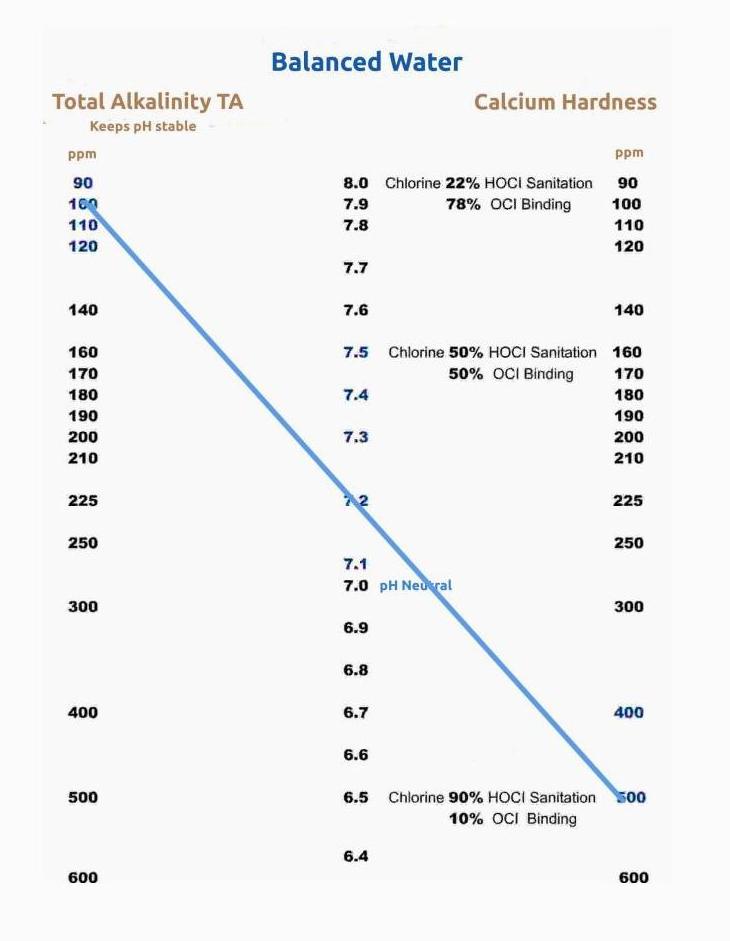
Balancing the water means the water is in balance, not too acidic, leaching minerals from pool walls or equipment or not too Alkaline (Basic), depositing surplus minerals and metals to pool walls and equipment.Developed in the USA one method is called the Watergram and the other The Langerier Saturation Index (LSI).
It measure the relation between the Total Alkalinity, PH and Calcium Hardness of pool water.
Within .3% of the levels in relation to each other, water is deemed balanced.
Balanced water is the set amount of certain mineral particles present in the water balanced to a certain PH to ensure that sanitation is kept optimal.
As a see saw.
Total Alkalinity TA - pH - Calcium Harness CH
TA 80-120 ppm pH 7.2-7.4 CH 300 - 600 ppm
at a certain water temperature with a certain amount of stabilizer.
Stabiliser Cyaunuric acid 5 - 20 ppm (0 in indoor pools and spa's)
First step in balancing the water.
Total amount of alkalinity particles in the water measured as parts per million.
Total Alkalinity controls pH fluctuations of the water.
Very important as sanitizing agents work best at certain pH ranges so keeping pH steady ensures optimal sanitation.
The standard is 90-110 parts per million for concrete pools and 110-150 ppm for fiberglass pools.
To raise Total Alkalinity by 10 ppm per 1000 liter of water add 15g Bicarbonate of Soda (baking soda), ph around 7,8.
To lower by 10 ppm per 1000 liter of water add 16ml of pool acid ( hydrochloric acid). Not more than a cup, 250 ml at a time.
Ph of the water tells us how acidic or alkaline the water is.
0 very acidic to 7 neutral to 14 very alkaline (basic).
The standard set 7,2 - 7,4 ensures bather comfort and how effective the sanitation chemicals will work. Keep in mind the balance of water in conjunction with Total Alkalinity and Calcium Hardness and the .3% variance. You could change TA or PH settings to be within the .3% parameter or cloudiness etc could occur. If you view the watergram there is a direct correlation (straight line ) between Total Alkalinity, PH and Calcium Hardness to get the water 100% balanced.
To raise PH by 0.1 per 1000l add 4g Soda Ash sodium carbonate Ph around 11
Aeration of the water splashing will also raise pH.
To lower, add hydrochloric acid or dry acid, sodium bisulphate according to the acid demand test. Not more than a cup, 250 ml at a time.
Too low the pH, acidic, your pool and equipment will be eroded and damaged.
To high, alkaline or basic, water molecules shed particles of matter absorbed. Scale builds up, water cloudiness prevails and metal particles if absorbed stains the pool walls.
Too high means that pool chemicals do not work effectively but at smaller effective percentages.
Sanitation agents as chlorine does not work effectively in water with a ph of 8 and above. At a pH of 6.5 chlorine is 90% effective, at a ph of 7.5 chlorine is only 50 % effective in sanitizing. Set your ph at 7.2.
Calcium Hardness is the amount of calcium particles in the water.
The standard is 300 - 600 ppm.
Too little and the water extracts calcium from the pool walls if concrete and too high scale forms and water could become cloudy.
To raise by 10 ppm add 15g Calcium Chloride per 1000l. Lowering the ppm will require reducing the pool water and adding new.
Another lowering method is to super Chlorinate, double to triple the amount of chlorine in the pool water to 10 ppm. At that level, calcium in the water will unbound and form cloudy particles. Switch the pump off for 12 hours and vacuum the settled particles to waste.
Stabiliser. Cyaunauric acid 7.5g per 1000l to raise by 10 ppm. Standard 5-20 ppm.
Chlorine dissipates very fast in sunlight due to ultraviolet rays. 35 % free chlorine per hour. On and on until the free chlorine is totally dissolved.
Stabilizer acts like sunscreen. It bounds chlorine in the water ensuring a gradual release instead of being eroded by the sun very quickly. This gradual release means that the sanitation effectiveness is also slower.
Adding stabiliser means less chlorine is depleted and chlorine costs curbed but it has to be weighed against the sanitation effectiveness of the chlorine.
Too much stabilizer prevents timely release of the chlorine and sanitation becomes less effective.
Studies indicating available free chlorine after one hour.
| Stabiliser ppm |
Free Chlorine % |
| 0 | 65 |
| 5 | 75 |
| 10 | 87 |
| 20 | 95 |
| 30 | 98 |
| 70 | 100 |
Studies showing 1.5 ppm chlorine sanitation effectiveness with stabiliser in minutes.
|
Stabiliser
ppm |
Sanitation Minutes |
| 0 | 8 |
| 10 | 12 |
| 20 | 30 |
| 30 | 50 |
| 40 | 65 |
| 60 | 75 |
Looking at the data a small amount of stabiliser could be beneficial in curbing chlorine costs. More than 15 ppm the effective sanitizing power of the chlorine becomes less to the point of being useless as a disinfectant.
Keeping in mind that chlorine as a sanitising agent becomes even more less effective with an increase of the waters pH value.
If you have an indoor pool or a spa do not use stabilizer as there are no uv rays to deplete the chlorine and for hot water you need maximum sanitation.
Many chlorine products contain stabilizer. Stabilizer builds up over time and is only reduced by new water.
Switch to non stabilized chlorine when 15 ppm stabilizer is attained.
So for an outdoor pool 15 ppm. In doors or a spa no stabilizer.
Water Temperature and Sun light
The sun emits ultra violet rays. These cause algae to spontaneous grow from matter in the water. The warmer the water the more algae flourish. Still or standing water in sunlight is a recipe for algae.
The higher the temperature the more water molecules move vibrate and interact with surrounding matter. ie the faster chlorine dissipates.
Total Dissolved solids.
The amount of other matter particles present in the water as metals, minerals, salt etc measured as ppm parts per million.
Sanitation is required to keep the water bacteria free ensuring bather safety and health.
Chlorine is the standard chemical used.
Effectively measured there should be between 1,5 - 3 ppm free chlorine in pool water.
Free chlorine means chlorine that can sanitize.
Chlorine does not sanitize as is.
When added to water it oxidize and forms Hypochlorous acid, HOCI, that itself partially dissociates forming Hydrochloric acid, OCI/HCI Hypochlorite ion.
Hypochlorous acid, HOCI, sanitize water, killing germs, bacteria and algae.
Hydrochloric acid, OCI/HCI hypochlorite ion, the less sanitizing but binding form of chlorine binds particles as urine and oils. These bound chlorine particles is called chloromites.
Free chlorine works and sanitize but the bound fixed chlorine, chloromites does not.
The percentages are determined by temperature and the pH of the
water.
The higher the pH the more OCl is created, less sanitizing of the water.
The lower the pH the less OCI is created, more sanitizing of the
water.
| pH | HOCI |
OCI |
Sanitation |
Binding |
| 6.5 | 90% | 10% | 90% | 10% |
| 7.5 | 50% | 50% | 50% | 50% |
| 8 | 22% | 78% | 22% | 78% |
Very important keep pH around 7.2.
HOCl ↔ OCl + H+ hypochlorous acid is in equilibrium with hypochlorite ion and hydrogen ion
The H+ is either liberated or used depending on the pH of the water. When it is liberated, it is then counted as part of the total hydrogen ions (H+) in the water which we call pH. The more H+, the lower the pH and the less H+, the higher the pH. This is just one example of a hydrogen ion source in the water.
As chlorine sanitize it is being depleted. It is also depleted by the sun's UV rays. In door and under cover pools are not affected by UV rays and chlorine in pools last longer and dosages much less.
Clean sanitized water does not smell. If there is a chlorine smell present it means that there are a large presence of chloromites, bound chlorine particles, in the water.
In order to remove the chloromites a double or triple dose of chlorine must be added to dissolve, oxidize, these bound particles.
Chlorine can be added to pool water in a few forms. Granular, as a floater and by way of a chlorination in salty water.
Granular means a cup of chlorine that is sowed over the pool surface, a floater is a container that contain chlorine in tablet form that release chlorine as it floats in the water.
Enough bags of salt is added to the water until a salt reading of 3500 is attained.
Too little salt and not enough chlorine is produced for effective sanitation
Too much and the chlorinator electrodes will be eroded and damaged.
Chlorination by way of electrical electrodes attached last in the the return water pipe line. As salty water pass the electrodes pure chlorine gas is produced. Chlorine gas bubbles emitting from the chlorination plates are visible when the pump is running.One key thing about salt system chemistry.
Chlorine gas is produced, which is acidic, and it neutralizes the high pH of the hydroxide produced.
This chlorine gas immediately converts to Hypochlorous acid, HOCI, the sanitizing form of chlorine in water and Hydrochloric acid, OCI/HCI hypochlorite ion, the less sanitizing but binding form of chlorine in water.
The same reaction as mixing chlorine with water.
Further Sanitation MethodsUltraviolet or UV light are invisible sun rays that falls in the light wave length spectrum between 10 and 400 nano meters.
Nano meter, Nm = billionth of a meter.
There are three different groupings according to their wavelength and harmfulness.
UVA 300 NM to 400 NM, least harmful.
UVB 290 NM to 320 NM causes sunburn but 95% is absorbed by the earths ozone layer.
UVC 100 NM to 290 NM, extremely harmful. Almost completely absorbed by the earths atmosphere. It is used as a disinfectant in food, air and water to render microorganisms useless by destroying their cell nuclei acids.
When water pass these rays micro organisms as bacteria are completely sanitized and oxidized.
This sanitized water enters the pool, combines and absorbs every thing in the pool water. So a sanitizing agent as chlorine is still necessary in the body of water for optimal pool sanitation.
Commercially two type of UV lights are available.
Light wave length 254 NM
Medium wave length 200 to 400 NM with this medium more effective as it kills of and destroy chloromites as well.
As time pass the lights weaken and should be replaced after a period of 12 months.
Ozone O3, a bluish lethal gas when mixed with water disinfects and oxidize all matter very effectively.
Air, Oxygen O2, molecules have two oxygen atoms. Ozone O3 molecules have three oxygen atoms.
Ozone is created when split Oxygen molecules are reforming.
Commercially two methods are available.
CD. Corona Discharge Ozone Generator and by UV, Ultra Violet Light.
The machine create a high electrical discharge in the form of a ring of energy.
Air, O2 molecules passing through are split. When these split molecules are reforming O3 ozone is created which is introduced to the pool water system.
Dry air, O2 molecules passing a uv light emitting rays light in the 185 nanometer range breaks up.
When these split molecules reform O3 ozone is created and introduced to the pool water supply.
An excellent disinfectant and oxidiser but as with an ordinary Uv machine the water is disinfected at contact point. So a little other disinfectant agent as chlorine is necessary in the pool water to keep the body of water sanitized.
Heating up pool water can be done various ways.
Two popular ways are by what is called solar heating and inverter heat pumps.
Pool water travels through plastic piping on the roof that is heated by the sun.
This does not generate much heat during winter or cloudy days, but it is free heat.
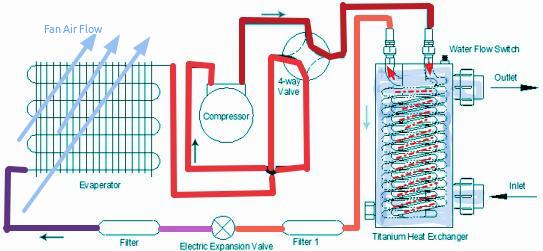
Pool water is pushed by the pool pump through a Titanium Heat Exchanger,
basically an in out flow water tank with coiled tubing inserted. In this
coiled tubing refrigerant flows.
a Sensor on the Titanium Heat Exchanger measures the water temperature and a Inverter controller automatically adjusts the operation capacity to a desired water temperature.
a Fan in the pool heat pump draws in the outside air and directs it over the evaporator.
This ambient air heats up the liquid refrigerant within the evaporator coil and the liquid becomes a gas. This warm gas refrigerant passes through a compressor and by pressure is heated to a very high temperature. This very heated gas pass through the condenser (Titanium Heat Exchanger) in the coil and heats up the cooler water flowing through the tank back to the pool. The hot gas refrigerant cools, reverts to liquid form again.
The whole process starts over again and continues until the water is
heated to the required temperature setting.
Aside from the electricity used to power the fan and compressor, an
inverter pool heat pump uses very little energy, making it one of the most
energy and cost-effective options available for heating a pool.
The latest pool heat pumps have a cop of up to 16. 1 Unit of energy produce 16 units of heat. Normal gas or electric heaters have a cop of not more than 1.
Water Testing
Testing strips works well in identifying the chemical composition of the pool water.
Take a sample of the water, elbow deep, dip in the strip for 2 seconds and compare to the coloring on the strip container. Then balance the water by adding the necessary measurement of chemicals.
Metal Stains
There could be numerous metals present in the water molecules.
Metals are more soluble at lower pH. When pH is raised metal
particles are expelled out of the water molecule and stains the
surrounding pool wall and floor. This can be contained and removed by
adding a metal remover agent binding metal particles that can be trapped
by the filter media and back washed later.
A filter media as Zeolite in place of silica sand also traps metal particles.
Ascorbic acid, Vitamin C is a good agent to remove metal stains.
Algae
Not a plant not fungal but plant like that supplies the earth and water wih 50-80% oxygen. Grows and survive by photosynthesis. Sunlight. Stores nutrients and provider of oxygen to fish and other aquatic life. Essential for life.
But to a pool owner a very unattractive green pool.
Major sources for algae are, spores, plants, grass and leaves in the water.
Ideal conditions stagnant water and sunlight. Algae forms immediately under these conditions.
Remedies if formed Algaecide kills algae, chlorine binds and suffocate it.
Healthy Pool Water
Keep the water balanced and sanitized at required levels.
Run the pump as long as possible during the day and a little after sunset without the pool cleaner attached.
Matter fallen in the water will be trapped in the the basket
filters for removal and smaller by the filter.
If a sand filter use a zeolite medium.
Keep filters clean by backwashing and rinsing weekly.
For algae present use an algaecide.
There are various parameters set by various parties for balancing water. Some so wide that you may be in the parameters stated by them but the water is not balanced within the parameters that is required for effective sanitation and clear water.
Balancing water could be tricky. Download a free app from orendatech.com
to assist with this. It guides you to correct amounts of chemicals to
balanced water.